Overview:
Energy conservation and environmental protection play an important role in our daily lives; and with the release of hybrid cars and electric cars that are price-friendly, people's awareness has been further enhanced. Both technologies use a large number of rechargeable batteries, of which high-quality, high-power lithium-ion battery cells represent the best solution to date. These batteries are widely used in notebook computers, cell phones, digital cameras, camcorders, and other portable devices, but productivity is not a major issue because these batteries have lower capacity, typically less than 5 amps per cell or group. (Ah). A typical battery pack consists of less than a dozen battery cells, so matching is not an important issue.
One way to achieve energy savings is to store electrical energy during off-peak hours to supplement the demand for electricity during peak hours. Batteries for vehicle or electrical energy storage have much higher capacities, typically a few hundred Ah. This is achieved by a large number of small battery cells or some high-capacity batteries. For example, a model of electric vehicle uses approximately 6,800 18,650 lithium-ion battery cells weighing up to 450 kg. For this reason, battery production requires faster manufacturing, higher efficiency, and more precise control to meet market price demands.
Lithium-ion battery manufacturing overview
Figure 1 shows the lithium ion battery manufacturing process. The battery formation and testing in the offline conditioning step not only has a great impact on battery life and quality, but also a bottleneck in the battery production process. 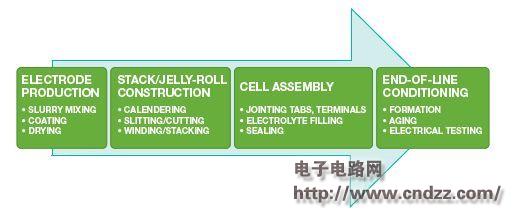
Figure 1. Lithium-ion battery manufacturing process
As far as current technology is concerned, it must be completed at the cell level, which can take hours or even days depending on the chemistry of the battery. A 0.1 C (C is battery capacity) current is typically used in the formation, so a full charge and discharge cycle will take 20 hours. The formation can account for 20% to 30% of the total cost of the battery.
Electrical tests typically use a 1 C charge current and a 0.5 C discharge current, so that one hour of battery charge time and two hours of discharge time are required for each cycle, and a typical test sequence includes multiple charge and discharge cycles.
Chemical and electrical tests have stringent accuracy specifications with current and voltage control within ±0.05%. For comparison, when charging batteries for portable devices such as cell phones and laptops, the accuracy may be only ±0.5% (voltage) and ±10% (current). Figure 2 shows a typical lithium ion charge and discharge curve. 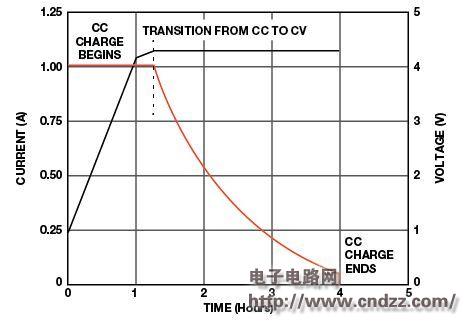
Figure 2. Charge and discharge curves for a typical lithium-ion battery (please read the PDF for details)
Copper Clad Steel ,Copper Clad Steel Highly Conductive,Copper Clad Steel Strand,Anti-Oxidation Copper-Clad Stee
changzhou yuzisenhan electronic co.,ltd , https://www.ccs-yzsh.com